|

Chemical and Physical Structure of Fatty Acids
30
April 2004
by Wyn Snow, Managing Editor
The
terminology surrounding fatty acids can be confusing.
We
hear about saturated, mono-unsaturated, poly-unsaturated, and trans
fats. There are monoglycerides, diglycerides, and triglycerides—which
our doctors tell us are a bad thing to have running rampant in our
veins and arteries. How do we tell which are the "good fats" and
which are the "bad" ones?
Looking
at the structure of fatty acids can clear up a lot of this confusion.
In the diagrams, the red circles are carbon atoms, forming the spine
at the center of the molecules. The blue circles are oxygen, and
the green are hydrogen.
The
following diagrams show a 2-dimensional rendering of a 3-dimensional
structure, as if the molecule has been mashed flat.
At
the core of all fatty acids: a chain of carbon atoms
One
of the simplest fats is butyric acid—found in butter. All fats
have a COOH acid at the beginning of the chain, also known as the
"alpha" end. The opposite end is called the omega (following the
Greek alphabet, which begins with alpha and ends with omega).
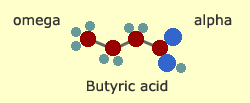
Butyric
acid is a saturated fat, which means that all the carbon bonds in
the middle of the chain are "filled" with hydrogen. It's also one
of the shortest fats, with only four carbon atoms.
A
smorgasbord of fats
Fatty
acids come in many lengths with many names. The three major omega-3s
are alpha-linolenic acid (ALA, the essential fatty acid that the
body cannot make) and the two fish oils eicosapentaenoic acid (EPA)
and docosahexaenoic acid (DHA). These "fancy names" for EPA and
DHA are based the Greek words for 20 and 22, which are the number
of carbon atoms in them.
Fatty
acids are the simplest of the fats. Glycerides occur when one or
more strings of fatty acids become attached to a glycerol molecule.
There can be one fatty acid string (monoglycerides), two (diglycerides),
or three (triglycerides) attached to the glycerol.
All
saturated fats are essentially straight, and the molecules can be
packed tightly together. This makes them relatively dense, and solid
at room temperature.
Unsaturated
fats have "missing" hydrogen
In
butyric acid and other saturated fats, each of the carbon atoms
in the middle of the chain is bonded with two hydrogen atoms. In
unsaturated fats, some of these hydrogen atoms are "missing." This
always occurs in adjacent pairs of carbon atoms, which then form
a double bond with each other. (Carbon atoms form 4 bonds with other
atoms. Oxygen forms 2 bonds and hydrogen 1.)
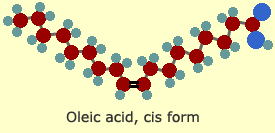
Oleic
acid is a common fatty acid found in most animal and vegetable fats.
It contains one double bond (at the bottom of the "v"). Note that
each of those carbon atoms is linked to only one hydrogen atom,
instead of two. Oleic acid is a mono-unsaturated fat, which means
it has only one double bond. Poly-unsaturated fats contain two or
more double bonds.
Fatty
acids can be short, medium, or long—containing anywhere from
4 to 28 carbon atoms in the chain. Butyric acid contains 4 carbon
atoms and is saturated with hydrogen atoms. The omega-3 called docosahexaenoic
acid (DHA), which is commonly found in fish oil, contains 22 carbon
atoms and 6 double-bond kinks, making it extremely polyunsaturated
indeed.
Because
unsaturated fats have this "kink" or bend, the molecules do not
stack together easily—so they stay fluid at room temperature.
Some mono-unsaturated fats, such as olive oil, will solidify when
cooled in the refrigerator. Poly-unsaturated fats, which have more
double-bonds and therefore more bends in their physical structure,
stay fluid even when refrigerated.
Why
trans-fats are so bad for us
When
plants or animals make unsaturated fats, they mostly build this
kinked "cis" form. However, food manufacturers discovered that bubbling
hydrogen through polyunsaturated oils created "partially hydrogenated"
fats that were less vulnerable to becoming rancid than the original
oils and therefore had a longer shelf life. These partially hydrogenated
margarines and shortenings are now present in almost all baked goods
and commercial peanut butter.
This
hydrogenation process also converts the bent "cis" form to a straightened
"trans" form, which looks like this:
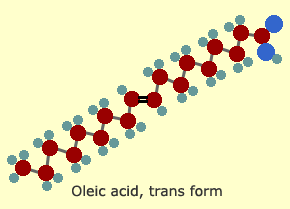
The
chemical structure is the same. It has the same number of carbon,
oxygen and hydrogen atoms, the same COOH acid at the alpha end,
and the double bond is in the same place—but now it's straight
instead of kinked.
The
body recognizes this chemical structure and tries to use it in the
same places and for the same purposes that it uses the bent cis
form. But the trans form stacks together just like saturated fats,
which sabotages the flexible, porous functionality the body needs
from unsaturates.
Exposure
to prolonged heat (as in deep fat frying) also creates trans fats
by loosening the double bond and allowing it to "flip" into the
straight form.
Poly-unsaturated
fats
In
poly-unsaturated fats, more than one hydrogen pair is missing. The
more pairs that are missing, the more kinks in the chain and the
more fluid the oil.
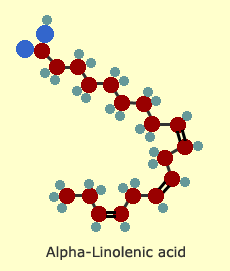
These
kinks in the structure make our cell membranes flexible and permeable,
allowing nutrients to enter the cell and waste products to leave.
When the body uses trans fats in place of the cis-form of unsaturates,
our cells can become insulin resistant, which can lead to type 2
diabetes.
Omega-3,
omega-6 and omega-9
Whether
an unsaturated fat is an omega-3 or -6 or -9 depends on the location
of the first kink or double bond. The acid end of the molecule is
called the "alpha", and its opposite is the "omega" end.
The
first double-bond kink in an omega-3 fatty acid occurs between the
third and fourth carbon atoms away from the omega end. For an omega-6,
between the sixth and seventh. And for omega-9, between the ninth
and tenth. Oleic acid (shown earlier) is an omega-9 fatty acid.
The
following simplified diagrams show the structure that is typical
of omega-3s and omega-6s.
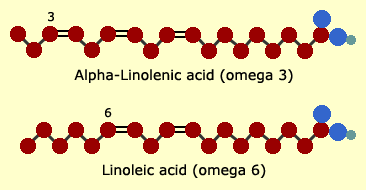
These
two fatty acids—alpha-linolenic acid (ALA, an omega-3) and
linoleic acid (an omega-6)—are the two essential fatty acids
that the human body needs and cannot manufacture. When sufficient
ALA is supplied in the diet, the body can make enough DHA and EPA
for the eicosanoids that form our metabolic "thermostat" system—or
the body can use DHA and EPA directly from diet as well.
The
bottom line on fats
Excessive
amounts of saturated fat in the diet have been linked to heart disease.
Trans fatty acids have been linked to increased insulin resistance
and type 2 diabetes—as well as heart disease. They appear to
be worse for our health than saturated fats, largely because the
body tries to use them in ways that evolution designed for unsaturated
fats. Consumption of trans fats also raises LDL cholesterol and
lowers HDL—the exact opposite of what one wants.
In
reading labels, steer clear of "partially hydrogenated" fats, because
these contain trans fats. In the future, food labels will be required
to display the trans fat content, making it easier to identify foods
that are bad for our health.
The
dietary move away from saturated fats to vegetable oils has supplied
plenty of the essential omega-6 fatty acid, linoleic acid, which
is present in almost all vegetable oils.
However,
obtaining enough of the omega-3 essential fatty acids has become
a challenge for most Americans. Eating more fish is one recommended
solution. Supplementing one's diet with omega-3 fish oils and/or
flaxseed oil is another.
Because
these omega-3 and -6 oils are polyunsaturated, they are especially
vulnerable to becoming rancid through exposure to light and heat.
Store such oils in the refrigerator—and when possible, purchase
brands with opaque containers. To be doubly safe with flaxseed oil,
keep it in the refrigerator even before opening the bottle.
|